how to draw molecular orbital diagram of no
Molecules consisting of two identical atoms are said to be homonuclear diatomic such as. Abstract TLDR Molecular orbital diagrams are a fantastic way of visualizing how molecular orbitals form using what we already understand about sigma and pi bonds.
Consider The Following Molecules No No And No Using The Molecular Orbital Theory How Do You Evaluate Them In Terms Of Bond Energy And Stability Quora
Draw the orbital diagram for the ion co2.
. 8 - Drawing Molecular Orbital Diagrams. Number of electrons in antibonding orbitals. Find the characters of the reducible representationfor the combination of.
Check out a sample QA here. Draw and explain all the properties of CO and NO with the help of molecular orbital diagram. Open an example of the MOdiagram package in Overleaf.
Electronic configuration of co molecule is. This command has two parameter in the example. Molecular Orbital Diagram of NO.
In the link above chem_mod said it was best to account for the negative charge of CN- by placing an extra electron on the nitrogen since it is more electronegative. The last electron is in fact in the 2b_1 antibonding MO so the bond order of NO has decreased by 12 relative to NO or CO. The region an electron is most likely to be found in a molecule.
Draw the mo diagram for n2. Hence the electronic configuration of N 2 ion will be. If non-linear yaxes of outer atoms point to central atom 3.
Draw another circle around the first shell. 1 2 iii A primal principle of these theories is that as atoms bond to form molecules a certain. So no electrons should be in that orbital and then finally once you have everything drawn fill the molecular orbitals according to the rules of electron configuration which would be Aufbau principle you have to build up Pauli exclusion you can only put two electrons in each orbital and hunds rule you have to fill or equal energy orbitals.
Sigma2s2Sigma2s2Pi2pxPi2py4Sigma2pz2 NO has 11 valence electrons. Assign x y zcoordinates zaxis is principal axis. 2 So the formula to find bond order is Bond order dfrac12 Number of electrons in BMO Number of electrons in ABMO Bond order dfrac12 8 2 Bond order dfrac12 6 Bond order 3 - N_2 molecules are diamagnetic with no unpaired electrons.
Creates bonds from overlap of atomic orbitals s p d and hybrid orbitals sp sp2 sp3 combines atomic orbitals to form molecular orbitals σ σ π π forms σ or π bonds. These can be further customized as you will learn in the next section. Molecules consisting of two non-identical.
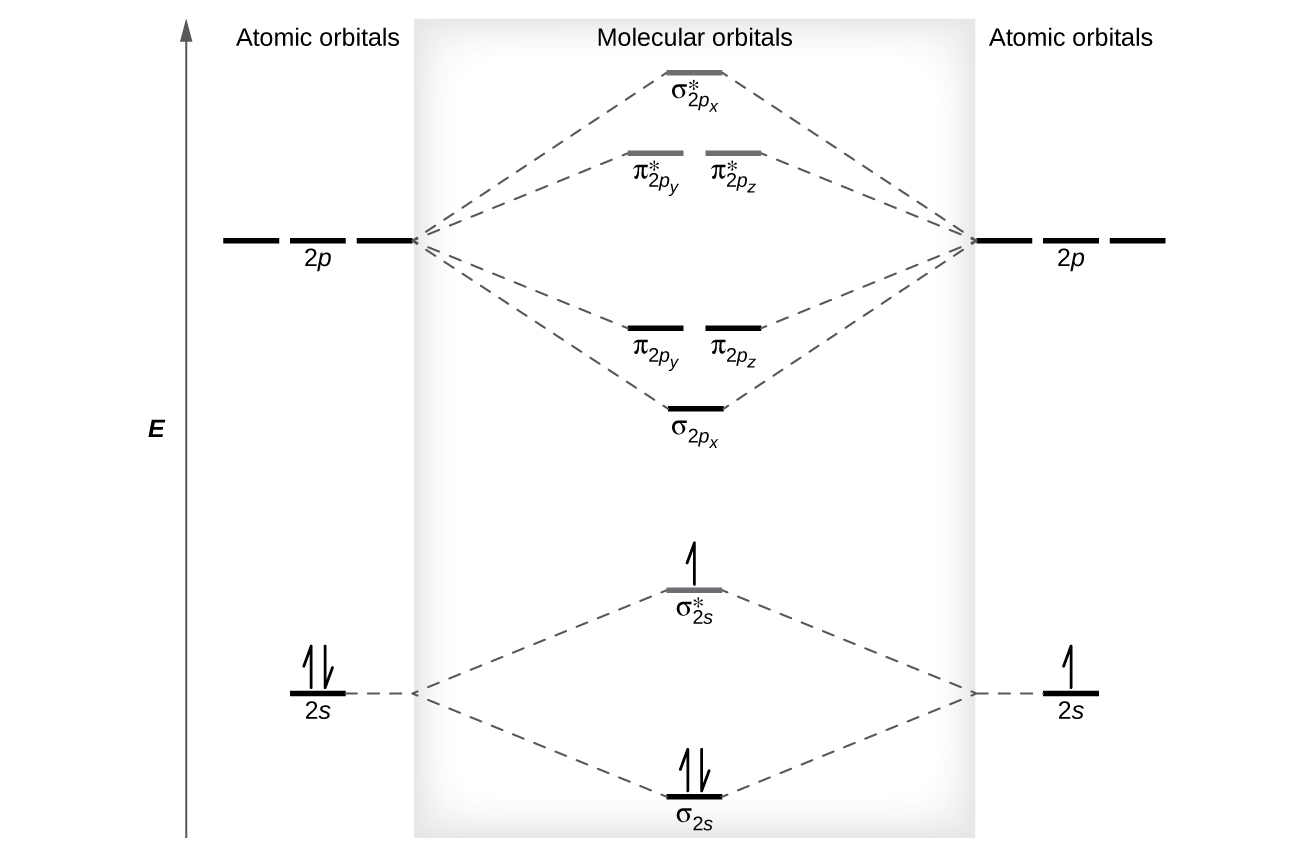
A molecular orbital diagram or MO diagram is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals LCAO method in particular. The molecular orbital diagram MO Diagram of N. An anti-bonding orbital is written as the bond with the star superscripted onto it.
H 2 N 2 O 2 and F 2. Answer 1 of 5. 1s 2s 2p Are the energy sub-levels to be drawn.
Molecular orbital diagram of no. Depending on if it is a homonuclear case where the bonding atoms are the same or a heteronuclear case where the bonding atoms are. For more informative Chemistry Lessons Subscribe DIGITAL KEMISTRY.
The bonding mos are the 2σ 1πx 1πy and 3σ which gives 2 2 2 2 8 bonding electrons. Visual tool in quantum chemistry. To obtain the bond order look at the molecular orbitals formed and decide whether they are bonding or antibonding.
The basic command to draw MO diagrams is atom. Determine point group of molecule if linear use D2hand C2vinstead of Dhor Cv 2. Molecular Orbital Theory Walsh diagram The Walsh diagram shows what happens to the molecular orbitals for a set of molecules which are related in structure.
Molecular orbital diagram of N 2 BO Nb-Na 10-4 3 Since all the electrons in nitrogen are paired it is diamagnetic molecule. Previous article Molecular Orbital Diagram of CO. N-O Hence the bond order of NO is either 25 or 35 depending on whether the last electron went into a bonding or antibonding MO.
The MO diagram of NO is. The alignment of the atom. σ 1 s 2 σ 1 s 2 σ 2 s 2 σ 2 s 2 Π 2 p x 2 Π 2 p y 2 σ 2 p z 2.
Draw out the MO diagram and label in the valence electrons. Part A Complete the MO energy diagram for the N2 ion by. Same as NO but add Pi2pxPi2py1 NO- has 12 valence electrons.
The proper notation is that molecular orbitals are written just by the kind of bond that the orbital creates. A MO is defined as the combination of atomic orbitals. Draw a circle around the nucleus.
Molecular orbital theory diagram for. Next article Qualitative and Quantitative Analysis Organic Chemistry. Same as NO but change 1 at the end to 2.
Consider the H 2 molecule for example. BO 12 bonding e- - antibonding e- 122222 - 21 colorblue25 And this should make sense because NO is isoelectronic with CO which has a bond order of 3. Drawing molecular orbital diagrams is one of the trickier concepts in chemistry.
Add up to two electrons to the first electron shell. I was just wondering if the same applied for molecules with a. In this case the difference is the H-X-H bond angle which decreases from o to 90 o Molecular Orbital Theory.
Draw the molecular orbital diagram for N22- Determine the bond order and if it is stable or not. Draw a small circle and write the symbol in the centre. Considers bonds as localized between one pair of atoms.
NO has 10 valence electrons. Draw the molecular orbital diagram for NO 3-Expert Solution. Instead the more electronegative element is drawn lower in.
How to draw an electron configuration diagram Find the element on the periodic table. Because the electronegativity of the two atoms are unequal the molecular orbital diagram will no longer be symmetric. Add up to eight electrons to the second shell.
Want to see the full answer. Click bellow CHANNEL LINK to subscribehttps. Considers electrons delocalized throughout the entire molecule.
Molecular Orbital Diagram of NO. Well the MO diagram for O_2 is. The content is presented using short focussed and interactive screencast.
Your MO diagram for NO should look like this.
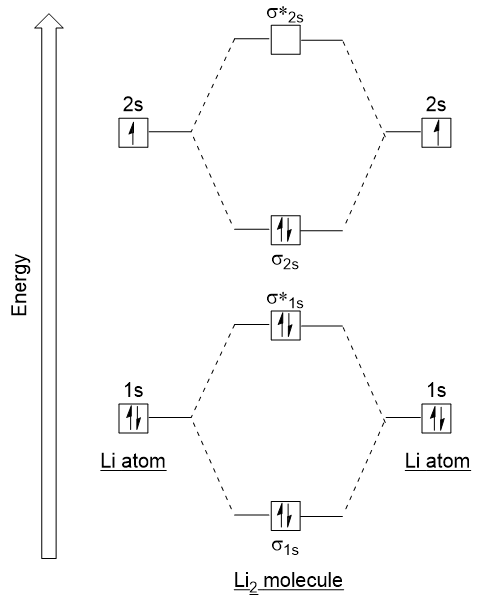
Molecular Orbitals Introductory Chemistry 1st Canadian Edition
Explain The Mo Diagram For No Molecule Sarthaks Econnect Largest Online Education Community
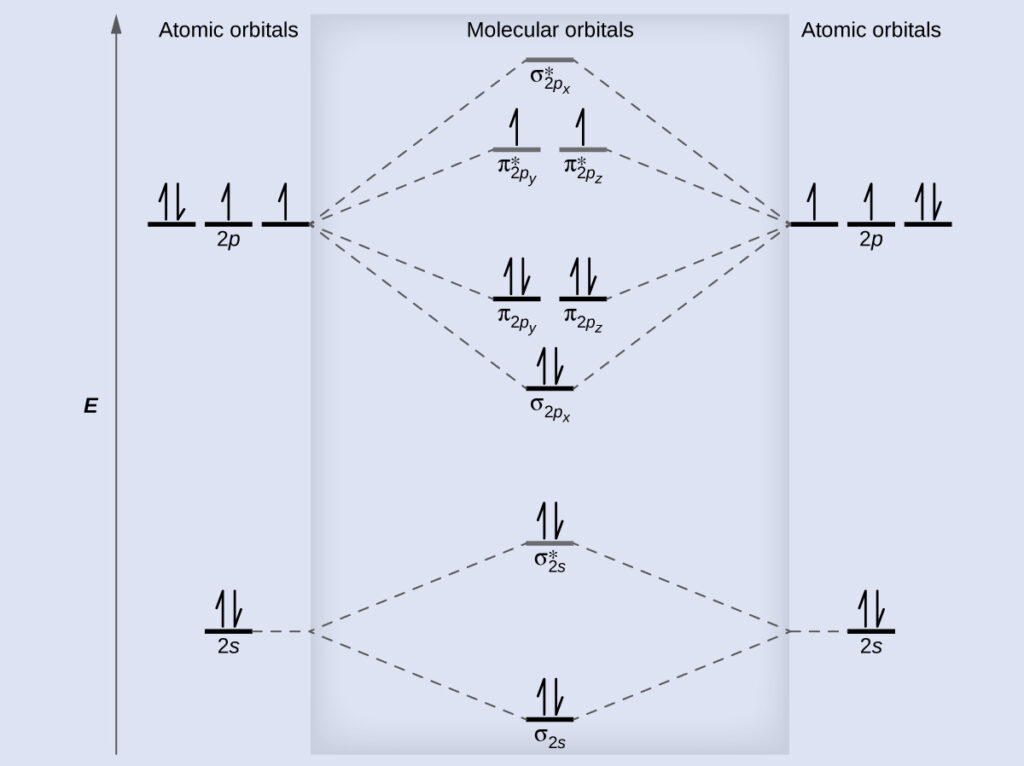
Molecular Orbital Diagrams Bond Order And Number Of Unpaired Electrons Chem Textbook

Molecular Orbital Diagram For No Download Scientific Diagram

Tikz Pgf Molecular Orbital Diagrams In Latex Tex Latex Stack Exchange
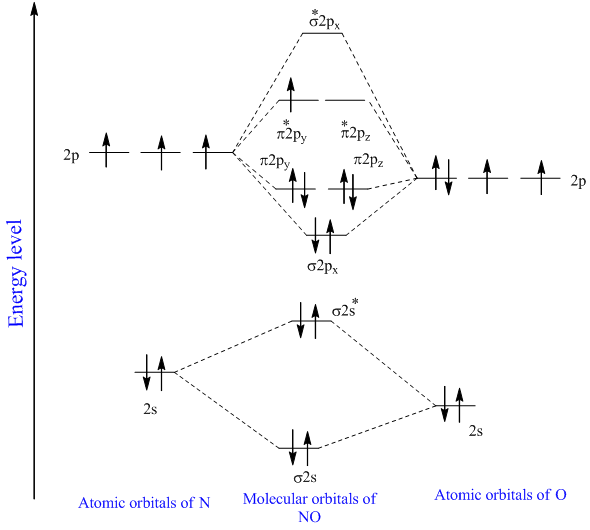
Solved Chapter 5 Problem 7p Solution Inorganic Chemistry 5th Edition Chegg Com

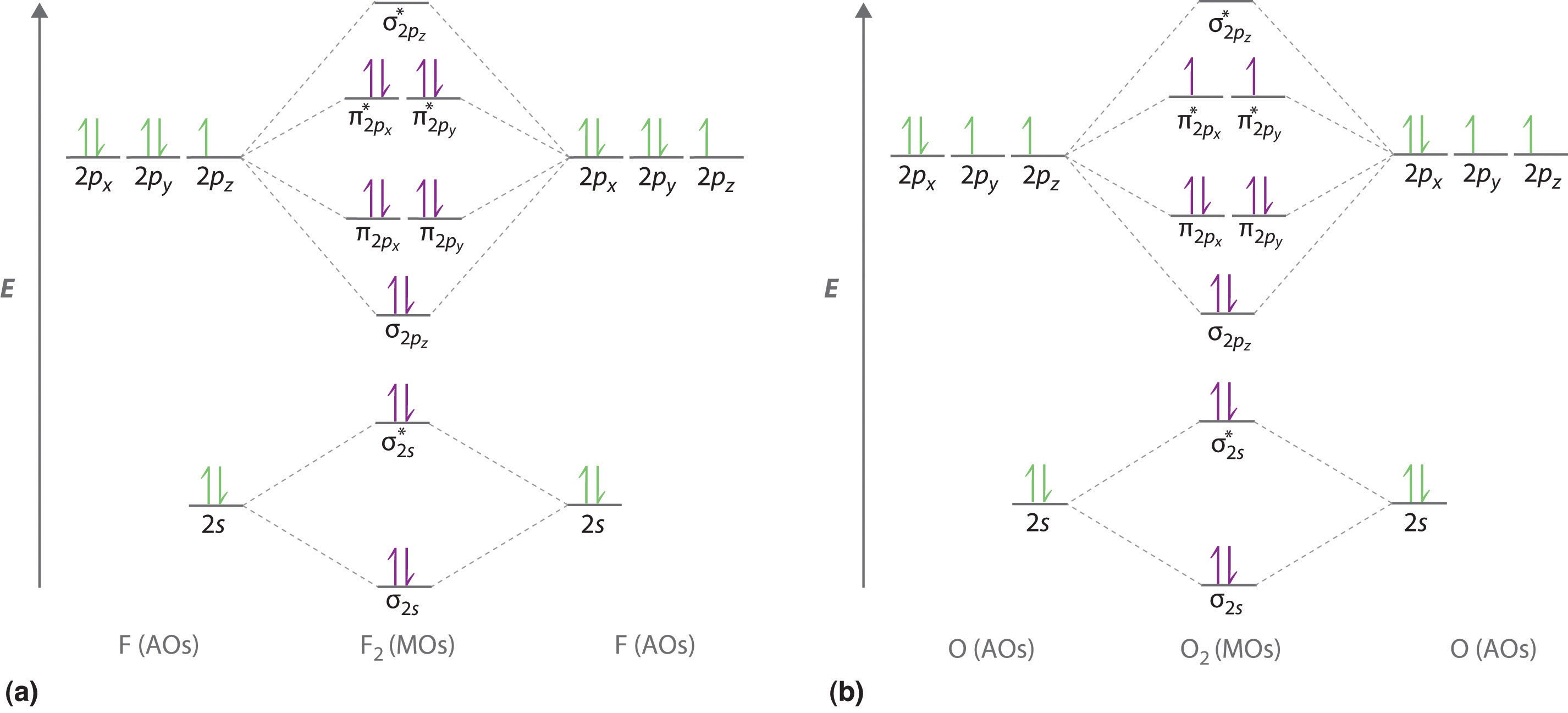
Draw The Molecular Orbital Diagram Of O2 And Calculate The Bond Order Is O2 Diamagnetic Or Paramagnetic Explain Your Answer Study Com

Molecular Orbital Theory Homodiatomics Use The Molecular Orbital Model To Fully Describe The Bonding In O2 O2 O2 And O22 Determine Which Of The Following Statements Are True And Which Are

Molecular Orbital Theory Does Peroxide Ion Have Any Unpaired Electrons Chemistry Stack Exchange

Delocalized Bonding And Molecular Orbitals

Mathematics Origins Of Molecular Orbital Diagrams History Of Science And Mathematics Stack Exchange
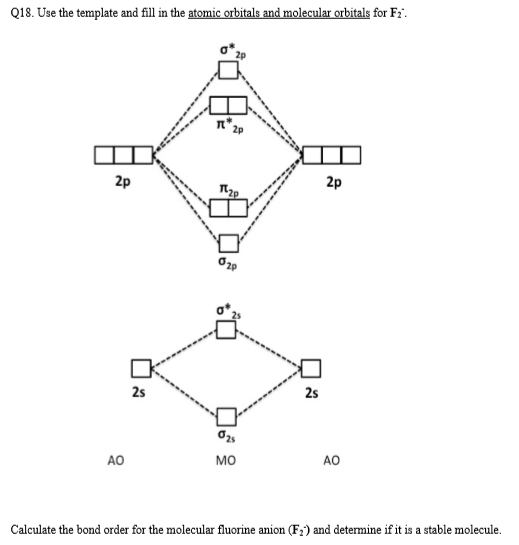
Solved Q20 Draw The Molecular Orbital Diagram For The Chegg Com

By Writing Molecular Orbital Configuration For No Co O2 Molecules Calculate The Bond Order And Also Determine Whether It Is Paramagnetic Or Diamagnetic Socratic
What Is The Molecular Orbital Energy Diagram Of Co Quora
2 2 Molecular Orbital Mo Theory Review Chemistry Libretexts
2 6 Molecular Orbital Theory Chemistry Libretexts

Construct Molecular Orbital Diagram And Determine Unpaired Electrons In O2 O2 Bn No Study Com
